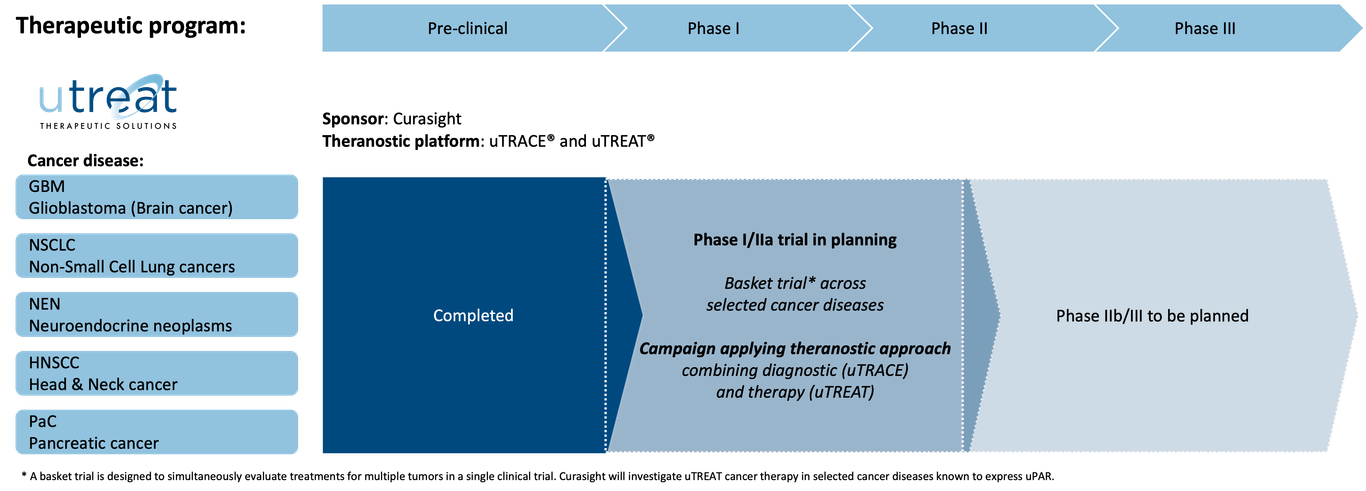
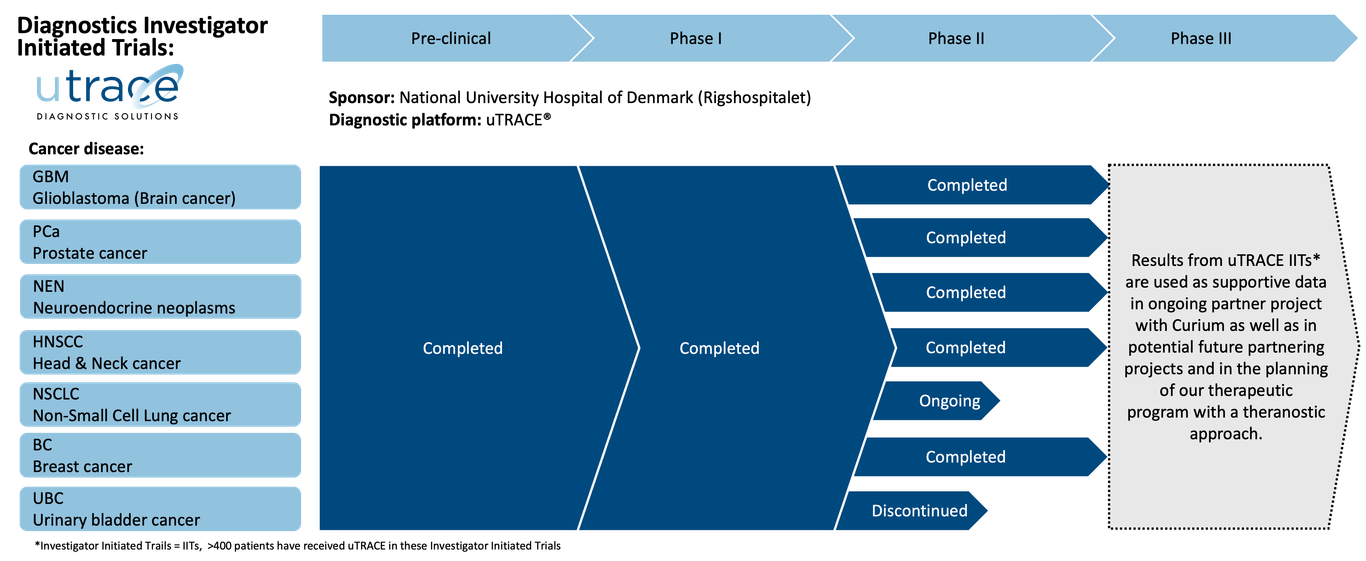
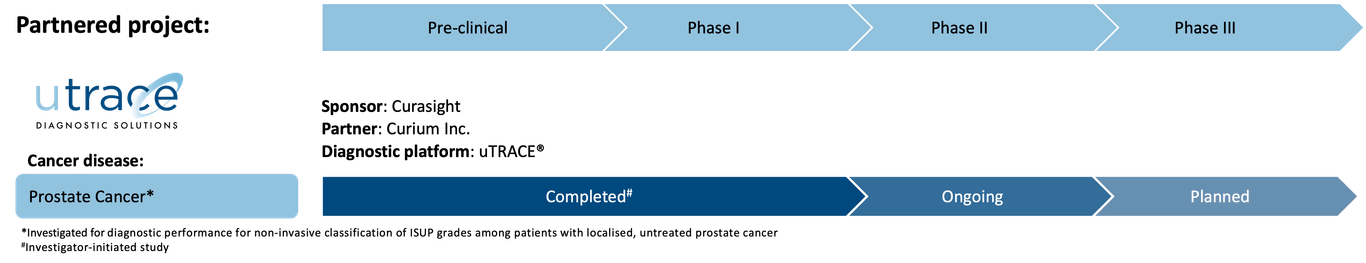
Pipeline
Curasight follows a theranostic approach using its uPAR radionuclide platform to improve both diagnosis and the treatment of certain types of cancer. The company is developing its uTREAT® therapeutic platform and its uTRACE® diagnostic platform in parallel and has a business development strategy to find partners for later stage clinical development and potential commercialisation.